Understanding Telomeres: Structure and Significance
Intro
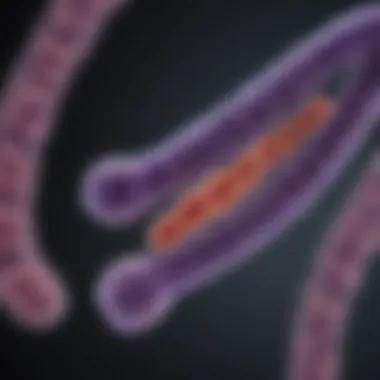
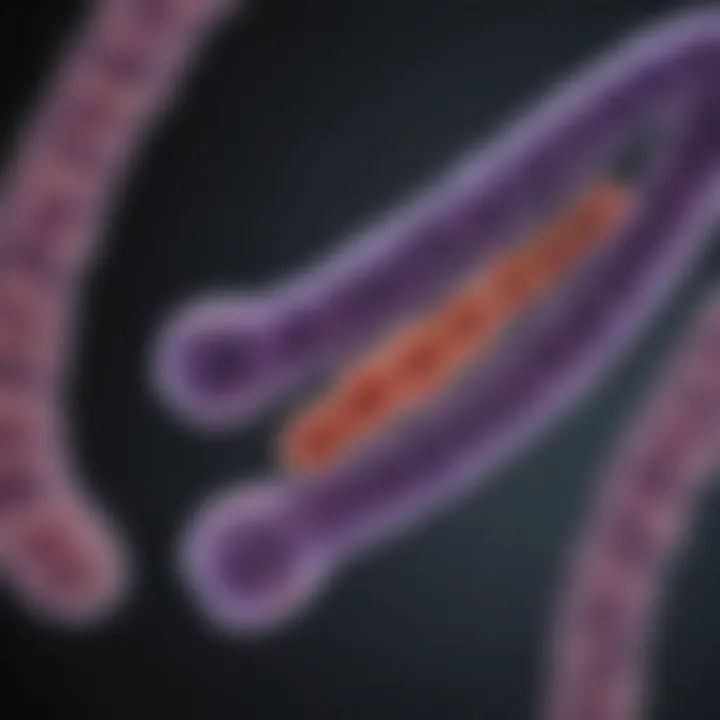
Telomeres are essential structures located at the ends of chromosomes. Their primary function is to protect the genetic information contained within these chromosomes during cell division. Despite their small size, telomeres play a significant role in cellular longevity, genomic stability, and overall health. Understanding telomeres is vital for deciphering complex biological processes, especially in relation to aging and various diseases.
Key Concepts and Terminology
Definition of Key Terms
- Telomere: A repetitive nucleotide sequence at the end of a chromosome that protects it from deterioration or fusion with neighboring chromosomes.
- Chromosome: A structure within cells that contains DNA, the hereditary material.
- Cellular Replication: The process by which a cell divides into two daughter cells, involving the duplication and distribution of its genetic material.
- Genomic Stability: The maintenance of the integrity of genetic information.
- Aging: The gradual accumulation of changes in an organism over time, including cellular deterioration.
Concepts Explored in the Article
This article will cover several key concepts, including:
- The structure and composition of telomeres.
- The mechanisms by which telomeres protect chromosomes during replication.
- Factors that can influence telomere length, such as lifestyle choices and environmental factors.
- The implications of telomere biology in relation to diseases like cancer and age-related disorders.
Findings and Discussion
Main Findings
Research has shown that telomeres shorten with each round of cellular division. This shortening is closely linked to the aging process. When telomeres become too short, cells can no longer divide, leading to cellular senescence. It is thought that this process contributes to many age-related conditions. The length of telomeres has also been associated with overall health, longevity, and disease susceptibility.
In addition, telomeres play a role in cancer. Many cancer cells have abnormally long telomeres, allowing them to replicate indefinitely. Understanding this can lead to potential therapeutic strategies that target telomere elongation in cancer treatments.
Potential Areas for Future Research
Further exploration of telomeres could lead to important advancements. Potential areas of research include:
- Developing therapies aimed at maintaining telomere length to combat aging.
- Investigating telomere length as a biomarker for chronic diseases.
- Understanding the role of telomeres in gene regulation and cellular function.
"Telomeres are not just protectors of the genome, they are also crucial players in the complex tapestry of cellular aging and disease progression."
Overall, understanding telomeres offers valuable insights into the fundamental aspects of biology, with potential implications for health and disease management. This research may one day illuminate paths for better health interventions and a deeper comprehension of the aging process.
Prelude to Telomeres
Telomeres are essential components of the cellular machinery that protect the integrity of chromosomes. The importance of telomeres lies in their role as a buffer at the end of the chromosomes. When cells divide, portions of DNA at the ends of chromosomes, known as telomeres, are not fully replicated. This leads to gradual shortening of telomeres over time, which is a key factor in aging and cellular senescence. Understanding telomeres is crucial for various fields, as they influence not only cellular lifespan but also contribute to disease mechanisms. The more we learn about these structures, the better equipped we become to address issues such as aging and cancer.
Definition and Discovery
Telomeres are repetitive nucleotide sequences located at the ends of linear chromosomes. They were first characterized in the 1930s when research began to shed light on chromosome structure. The term "telomere" itself is derived from the Greek words "tele," meaning end, and "meros," meaning part. Each time a cell divides, the telomeres shorten, which eventually leads to the cell's inability to divide, thus inducing senescence. The discovery of the enzyme telomerase in the 1980s was pivotal, as it was found to maintain telomere length in certain cells, such as stem cells and germ cells. This finding opened up a range of research avenues into how telomerase could be manipulated for therapeutic purposes.
Importance in Chromosome Stability
Telomeres serve critical functions in maintaining chromosome stability. They prevent the ends of chromosomes from being recognized as broken DNA by the cellular repair machinery. This is crucial because if the ends were mistakenly repaired, it could lead to chromosomal fusions, instability, or even cancer. Additionally, telomeres play a significant role in the regulation of cellular aging. When telomeres become too short, the cell can no longer divide safely and enters a state known as senescence. This is a natural safeguard against uncontrolled cell proliferation, but excessive senescence can contribute to tissue aging and various age-related diseases. Therefore, understanding telomeres is not only relevant for cell biology but also has implications in aging research and therapeutic strategies.
Structure of Telomeres


Understanding the structure of telomeres is essential for grasping their role in health and disease. Telomeres not only safeguard the genetic information stored in chromosomes but also influence cellular aging and stability. Their architecture is a fusion of repetitive DNA sequences and proteins, forming a specialized structure at each end of the chromosomes. Each specific element of this structure plays a crucial part in telomere function.
Telomeric Repeat Sequences
Telomeres consist of tandemly repeated DNA sequences, notably 5'-TTAGGG-3' in humans. These repeat sequences can extend for several thousand bases. The repetition provides a buffer against the loss of important genetic information during cellular division. When cells divide, DNA replication cannot completely duplicate the ends of linear chromosomes. The telomeric repeats shorten with each replication, but having these repetitive sequences means vital coding regions of genes are preserved. This safeguard is critical for maintaining functional integrity over time.
Additionally, the arrangement of these repeats results in a unique structure that contributes to telomere maintenance and function. A loss in the telomeric repeat length can trigger cellular senescence, affecting normal cell function and longevity. In this way, the telomeric repeat sequences are instrumental in regulating how cells respond to stress and aging.
Proteins Associated with Telomeres
Many proteins are associated with telomeres, each playing distinct roles in the telomere's integrity and functionality. Core components include the shelterin complex, which consists of six different proteins: TRF1, TRF2, POT1, TPP1, RAP1, and TMPy. These proteins interact specifically with the telomeric DNA and serve a variety of crucial functions:
- Protection: Shelterin proteins shield telomeres from being mistakenly repaired as damaged DNA.
- Regulation: This complex helps regulate telomerase activity, the enzyme responsible for adding telomeric repeats in stem cells and cancer cells.
- Maintenance: They assist in maintaining telomere length and structure, ensuring genomic stability during cell division.
The coordinated action of these proteins ensures telomeres do not undergo abnormal fusion events or degradation, thereby maintaining chromosome integrity. Recent research continues to highlight how the dysfunction of these proteins can lead to diseases, including cancer.
Telomere Cap Structure
The cap structure of telomeres is pivotal for their protective function. Composed of a looped structure known as a T-loop, the single-stranded overhang of the telomere folds back and invaginates into the double-stranded segment. This formation is critical for preventing DNA damage responses that could lead to cellular stress or apoptosis. The T-loop, along with the association of shelterin proteins, plays a dual role:
- Signal Prevention: It prevents the DNA repair machinery from erroneously recognizing telomeres as breaks in the DNA strand.
- Stability Enhancement: The cap structure maintains telomeres' stability by providing resilience against nucleolytic degradation.
Contrary to merely acting as a protective covering, the caps facilitate interactions between telomeres and other cellular processes, further emphasizing their importance in maintaining a healthy cellular environment.
In summary, the structural complexity of telomeres is fundamental to their ability to sustain chromosomal integrity and protect genetic information, intertwining their relevance significantly with cellular aging and disease.
Functions of Telomeres
Telomeres serve essential functions that are critical to the integrity and stability of chromosomes. These functions not only protect genetic material but also play a significant role in cellular physiology and aging. Understanding how telomeres operate can provide insights into numerous health conditions and their underlying mechanisms. The three primary functions of telomeres are protecting chromosomal ends, facilitating cellular replication, and maintaining genomic integrity.
Protecting Chromosomal Ends
Telomeres act as caps at the ends of chromosomes, preventing the loss of vital genetic information during cell division. Without these protective structures, chromosomes would deteriorate, leading to genomic instability. Telomeric DNA is repetitive and non-coding, which minimizes the risks associated with the end of chromosomes. Each time a cell divides, a small portion of telomeric DNA is lost. This protective nature is crucial for cells to maintain stability and prevent unwanted fusion between chromosomes. In essence, telomeres function as the "off switches" for the cellular aging process, which is vital for healthy cellular mechanics.
"Telomeres are not merely caps; they are gatekeepers of chromosomal health."
Role in Cellular Replication
During cellular replication, DNA is duplicated to ensure continuity for daughter cells. However, DNA replication is not flawless due to the limitations of the enzyme DNA polymerase, which cannot fully replicate the ends of linear DNA strands. Telomeres play a key role in addressing this issue by providing a buffer zone. The progressive shortening of telomeres is a natural consequence of replicative aging. When telomeres reach a critically short length, the cell enters senescence or apoptosis. Thus, they serve as biological clocks that inform the cell about its replication limits, contributing to the regulation of cell division.
Genomic Integrity Maintenance
The maintenance of genomic integrity is one of the primary functions of telomeres. They safeguard the chromosomes against damage caused by oxidative stress and other environmental factors that can lead to mutations. By providing a protective environment at the ends of chromosomes, telomeres help mitigate the risk of chromosomal aberrations that can occur when telomeres are compromised. This function is particularly relevant in the context of cancer biology, where malfunctioning telomeres result in genomic instability. Understanding telomeres contributes to the broader knowledge of how genetic diseases and cancers can arise when the mechanisms governing chromosome integrity fail.
In summary, the functions of telomeres extend beyond simple protection; they are intricate components essential for maintaining cellular and genetic integrity. Their roles are interconnected and vital for understanding a wide array of biological processes.
Telomere Length and Aging


The relationship between telomere length and aging is a significant area of research in genetics and cellular biology. Telomeres, the repetitive nucleotide sequences at the ends of chromosomes, serve a critical function in preserving chromosomal integrity. As cells divide, telomeres become progressively shorter, which has implications for cellular aging and overall health. Understanding this connection can provide valuable insights into aging processes, age-related diseases, and potential therapeutic approaches.
Telomere Shortening Mechanism
Telomere shortening is largely a consequence of cellular replication. Each time a cell divides, its DNA is copied, but the enzymes responsible for this process cannot fully replicate the ends of linear DNA strands. This leads to the gradual loss of telomeric repeats. Several factors influence how rapidly telomeres shorten, including:
- Cell type: Certain cells, such as stem cells, can maintain their telomeres through the activity of the enzyme telomerase. In contrast, somatic cells often lack this enzyme, leading to accelerated telomere shortening.
- Replication: The frequency of cell division plays a crucial role. Tissues that regenerate frequently, like skin and blood, may experience faster telomere loss compared to less active tissues.
- Environmental factors: Stress, poor nutrition, and exposure to toxins may contribute to faster telomere attrition.
This shortening mechanism is essential for understanding how telomeres act as a biological clock, signaling cellular senescence when their length reaches a critical threshold. Short telomeres can lead to genomic instability and increased risk of age-related diseases.
Impact of Telomere Length on Aging
Telomere length is often considered a biomarker for biological aging. Shortened telomeres have been associated with various age-related conditions such as heart disease, diabetes, and certain cancers. The implications of this phenomenon are profound:
- Cellular senescence: When telomeres reach a critically short length, cells may undergo senescence, stopping their division. This process contributes to aging-related tissue dysfunction.
- Inflammation: Short telomeres can lead to increased inflammation, which is linked to many chronic diseases. Inflammation itself can create a feedback loop, further accelerating telomere shortening.
- Predictive value: Measuring telomere length has the potential to predict health outcomes in aging individuals, offering insights for both prevention and therapy.
Research indicates that maintaining telomere length may promote longevity and decrease susceptibility to age-related diseases.
In summary, the length of telomeres is a critical factor influencing aging. Telomeres not only protect chromosomes but also play a crucial role in cellular health. Understanding their dynamics can pave the way for future interventions aimed at enhancing healthspan and combating age-related disorders.
Telomeres and Disease
Telomeres play a critical role in human health, influencing various diseases, most notably cancer. Their structure and function directly affect cellular aging and genomic stability. As telomeres shorten over time, they can lead to cellular malfunctions. This presents a clear link between telomere length and the onset of diseases, emphasizing the importance of studying telomeres to understand pathologies better.
Telomerase Enzyme in Cancer
The telomerase enzyme has gained attention in cancer research. In normal somatic cells, telomerase is virtually inactive, resulting in telomere shortening as cells divide. However, in many cancer cells, telomerase activity is reactivated, allowing these cells to maintain telomere length and avoid senescence. This evasion from cell death enables uncontrolled cellular proliferation.
Researchers are investigating targeting telomerase as a therapeutic strategy to combat cancer. Current studies focus on the potential to inhibit telomerase activity in tumors. By doing so, it may be possible to induce telomere shortening in cancer cells, leading to cell death. This approach represents a promising avenue for future cancer treatments.
Associated Genetic Disorders
Certain genetic disorders also display a connection with telomere dysfunction. For instance, dyskeratosis congenita is a rare genetic condition linked to mutations in genes responsible for telomere maintenance. Patients with this disorder exhibit very short telomeres and often face severe health challenges. Individuals with this condition suffer from skin changes, bone marrow failure, and an increased risk of cancers. The study of such disorders provides valuable insights into how telomere length can impact cellular health and function. Understanding these mechanisms can inform strategies for diagnosis and management of related genetic conditions.
Telomeres in Cardiovascular Diseases
The association between telomere length and cardiovascular diseases has sparked interest in the medical community. Studies have shown that shorter telomeres correlate with an increased risk of heart-related issues. This relationship suggests that telomere shortening is not just a marker of aging but a contributing factor to disease development.
Research indicates that oxidative stress and inflammation may accelerate telomere shortening, linking lifestyle factors such as diet and exercise to telomere health. By understanding these connections, interventions aimed at preserving telomere length could potentially reduce cardiovascular risk.
"Maintaining telomere length may provide a key to preventing various diseases and promoting longevity."
Overall, telomeres serve as important biological markers in understanding disease mechanisms, especially in cancer, genetic disorders, and cardiovascular health. Continued research in this area promises to expand our understanding and potentially lead to novel therapeutic interventions.
Current Research on Telomeres
Research on telomeres is evolving rapidly and has significant implications for biology and medicine. Understanding telomeres substantially contributes to our grasp of cellular aging and disease development. Insights gained from telomere studies can lead to better health strategies and innovative treatments, making this field invaluable.

Advancements in Telomere Biology
Current studies have delved into the molecular dynamics of telomeres. Researchers are actively examining variations in the telomeric DNA sequence and its consequences on telomere stability and function.
- CRISPR Techniques: The use of CRISPR technology has shown promise in modifying telomere length, which may help in correcting genetic disorders related to telomere attrition.
- Biomarkers for Aging: Some studies propose that telomere length could serve as a biomarker for aging. Newer methodologies can measure telomere length more accurately, allowing better prediction of age-related diseases.
- Telomere-Associated Proteins: Recent discoveries have identified various proteins involved in telomere maintenance. Understanding these proteins can further unravel telomere dysfunction, linking it with diseases like cancer.
These advancements not only clarify telomere biology but also indicate how they can influence future therapies and diagnostics.
Telomeres as Therapeutic Targets
The therapeutic potential of targeting telomeres is becoming a focal point in contemporary research.
- Cancer Treatments: Telomerase inhibitors are being studied to obstruct cancer cell proliferation. Cancer cells often hijack the telomerase enzyme to maintain their telomeres, allowing for infinite divisions. By inhibiting telomerase, researchers aim to limit cancer growth.
- Drug Development: New drug candidates are focusing on telomere biology to promote telomere extension while maintaining genetic stability in non-cancer cells.
- Gene Therapy: Approaches utilizing gene therapy aim to enhance telomere function directly, potentially reversing some effects of aging.
"Targeting telomeres offers a unique approach for therapeutic advances in oncology and regenerative medicine."
As research progresses, the immediate and long-term benefits of manipulating telomeres in treatments become clearer. Efforts concentrate on not only understanding telomere biology but also finding methods for practical applications in health management and disease prevention.
Future Directions
The field of telomere research continues to evolve, offering both exciting prospects and significant challenges. Understanding future directions in telomere biology is paramount, as it holds the potential to unlock new therapeutic avenues for age-related diseases and cancer. As scientists delve deeper into the intricacies of telomeres, several key areas require attention. Through focusing on the preservation of telomere length, we may improve cellular longevity and health outcomes.
Possible Interventions to Preserve Telomere Length
Research into telomere length preservation presents various strategies that could lead to potentially transformative healthcare solutions. Here are some interventions considered worthwhile:
- Lifestyle Modifications: Evidence suggests that diet, exercise, and stress management can influence telomere length. For instance, a balanced diet rich in antioxidants might help mitigate oxidative stress, which contributes to telomere shortening. Regular physical activity has also been linked with longer telomeres.
- Pharmaceutical Approaches: Some compounds are under investigation for their ability to activate telomerase, the enzyme that lengthens telomeres. For example, TERT gene therapy is being studied as a method to boost telomerase activity in specific cell types.
- Nutraceuticals: Natural products may also play a role in telomere maintenance. Certain vitamins and supplements, such as vitamin D and omega-3 fatty acids, show potential in supporting telomere stability.
Ultimately, detecting and evaluating changes in telomere length through comprehensive approaches can reveal insights into the aging process and guide us towards effective interventions.
Research Trends and Emerging Technologies
As the understanding of telomeres advances, new research trends and technologies emerge, shaping the future landscape of this field. Some notable trends include:
- CRISPR and Gene Editing: The CRISPR-Cas9 technology allows precise modifications to the genome, including telomerase genes. This method offers remarkable possibilities to investigate telomere functions and their implications in health.
- Longitudinal Studies: There is increasing interest in long-term studies that track telomere length across different populations. Such studies provide valuable data on how lifestyle, genetics, and environmental factors interplay with telomere dynamics over time.
- Biomarker Development: Identifying telomere length as a biomarker for various diseases will facilitate early diagnosis and potentially tailor interventions. Innovations in monitoring and measuring telomere length efficiently could enhance their clinical relevance.
"Exploring the future of telomere research opens doors to mitigating age-related decline and understanding cancer biology more profoundly."
Epilogue
The conclusion of this article is pivotal in encapsulating the core concepts surrounding telomeres. It emphasizes the significance of telomeres from both a structural and functional perspective. Understanding telomeres is not merely an academic exercise; it holds crucial implications for aging, disease prevention, and potentially therapeutic interventions. By summarizing the main points discussed throughout the article, we can appreciate the interrelatedness of telomere biology with critical biological processes in organisms.
Summary of Key Points
In summarizing the discussion about telomeres, several key points emerge:
- Telomeres are protective structures: Located at the ends of chromosomes, they prevent the loss of genetic information during cell division.
- Telomere shortening is inevitable: Each time a cell divides, telomeres become shorter, leading to eventual cellular senescence.
- Telomeres and aging: There is a correlation between telomere length and the aging process. Shorter telomeres are associated with age-related diseases.
- Telomere maintenance mechanisms: The enzyme telomerase can extend telomeres, primarily active in stem cells and cancer cells.
- Research advancements: Ongoing research seeks to unlock potential therapies targeting telomeres to enhance health span and combat diseases associated with telomere dysfunction.
The Importance of Ongoing Research in Telomere Biology
Research in telomere biology is essential due to its broad implications. As the study of telomeres progresses, researchers can better understand how telomere dynamics influence health and disease. The development of potential interventions to maintain telomere length opens avenues for addressing age-related conditions and malignancies. Furthermore, as technology evolves, our ability to study telomeres in real-time will improve, yielding richer insights.
Continuous investigation into telomere biology not only enhances our understanding of fundamental processes in life but also paves the way for medical advancements that could redefine health management.







